-40%
Dental Slow Low Speed Handpiece Wrench Contra Angle E-type Air Motor 2 Hole 1:1
$ 15.3
- Description
- Size Guide
Description
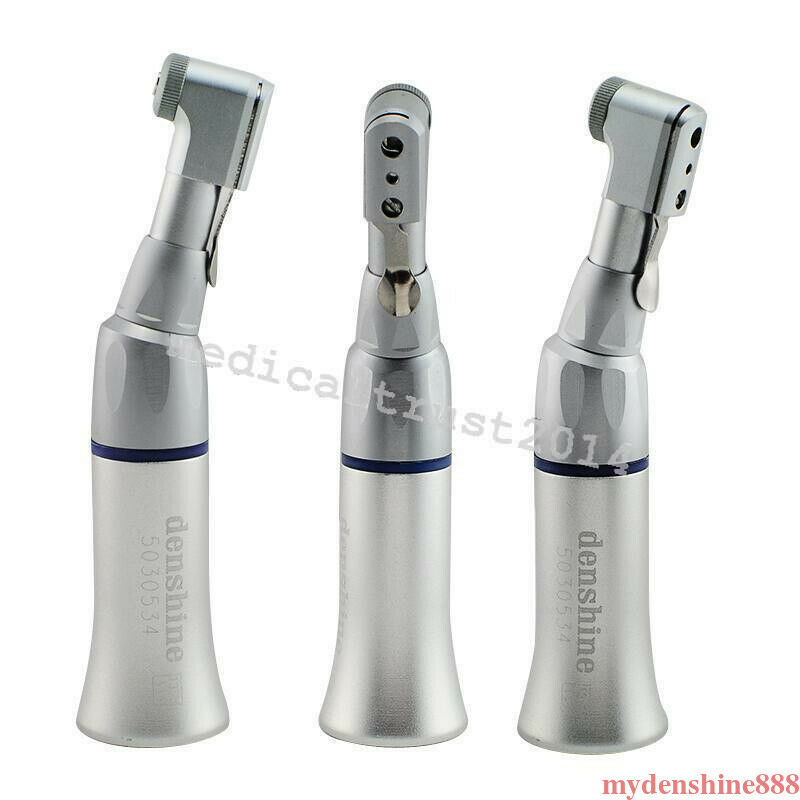
NEW Dental Slow Low Speed Wrench Type Handpiece Contra Angle Latch BurSKU:180170
Contra angle handpiece
- Low Speed 22,000-27,000 rpm.
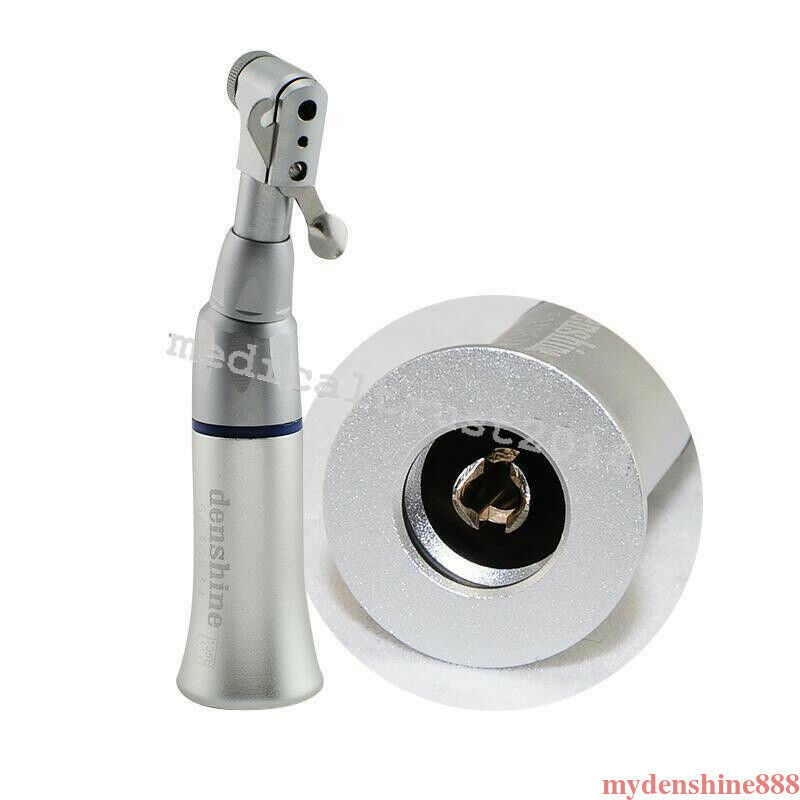
- The shank of the instrument mounted therein is 2.35 mm
- Fastening of the instruments with a turning latch
- Autoclavable 135°C
- For any lab or E-type motors
Warranty:
- Long Life-Span
- One Year Manufacturer Warranty
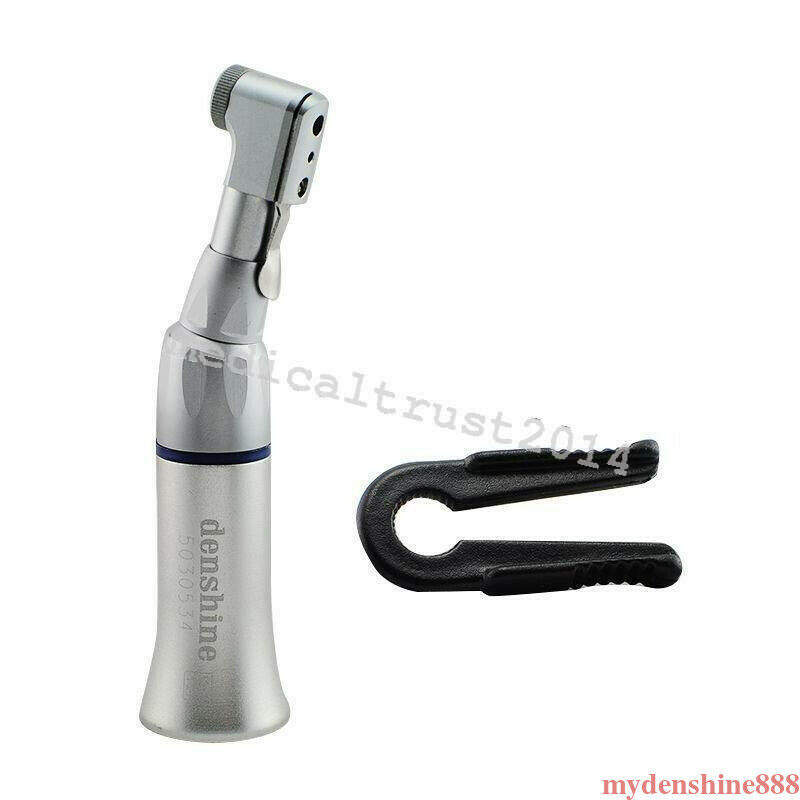
Packing List:
- Contra Angle handpiece: 1 each
- English manual: 1 each
SKU:180174
Features:
- Intended for dental clinical treatment and prevention
- Suitable for any E-type headpiece accords with ISO standard
- Suitable for both steam and chemical autoclave
- European CE certificate
Air Mortor
- Supply air pressure: 245-392KPa (2.5-4kgf/CM2)
- 1:1 Direct Drive
- 2 holes
- Max speed 20,000 min-1
Specifications
Air-in Pressure(kg/c㎡)
Motor Rotation(rpm)
Air Wastage:(N1/min)
2.5
22,000
42
3
25,000
51
4
27,000
72
Warranty:
- Long Life-Span
- One Year Manufacturer Warranty
Packing List:
- E-type air motor 2-Hole: 1 each
- English manual: 1 each
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.(CICI-China-86-18328503374)
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892